Liquids and Vapors
Liquids and Vapors
If a pure liquid is heated at a constant pressure there is a fixed
temperature at which bubbles of vapor
form in the liquid; this phenomenon is known as boiling. The states of substance at this condition
represents a point on the properties diagram, known as boiling point; e.g. point 1. A slight addition of heat
to the liquid at this state changes some of it into vapor.
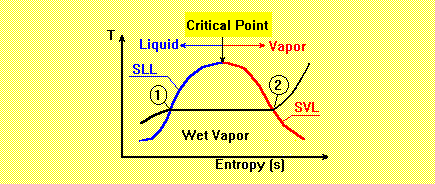
Saturated liquid line, SLL, is formed by connecting a series of boiling points.
Boiling temperature known
also as saturation temperature, T, for a pure liquid is only a function of pressure, P, i.e.
T2 = T1 = f(P)
Vaporization continues by further heat supply to the system until no liquid is left. This state is known as
dry saturated vapor, e.g. point 2. If the system is slightly cooled at this state, then droplets of liquid will
begin to form.
Connecting a series of points at dry saturated vapor builds a line, known as saturated vapor
line, SVL. The
state of substance between saturated liquid and dry vapor is called wet vapor.
Further heating of a dry saturated vapor at constant pressure causes a rise of vapor temperature and it
becomes superheated. The state of substance is completely defined by its pressure and temperature if it
is in liquid or superheated vapor phase i.e.
h = f1(P,T) = Specific enthalpy
v = f2(P,T) = Specific volume
s = f3(p,T) = Specific entropy
These properties for different substances are either tabulated or can be calculated by certain equations,
e.g. IFC formulation for water and steam properties. The state of wet vapor can not be defined by just
pressure and temperature until one other property is given. The condition or quality of wet vapor is often
defined by its dryness or wetness fraction.
dryness fraction, x = the mass of dry vapor in 1 kg of the mixture,
and,
wetness fraction, 1 - x = the mass of liquid in 1 kg of the mixture.
For wet vapor with the dryness fraction, x,
h = (1-x) hf + x hg
v = (1-x) vf + x vg
s = (1-x) sf + x sg
where f and g indicate the property of the substance at saturated liquid and dry saturated vapor states
respectively. The heat supplied to the liquid for the complete phase change is called the specific enthalpy
of vaporization.
hg - hf = hfg
Related topic:
Copyright © 1998 Taftan Data. All Rights Reserved